Galactose-functionalised PCL nanofibre scaffolds to attenuate inflammatory action of astrocytes in vitro and in vivo†
Abstract
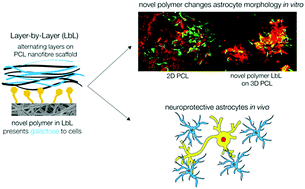
Astrocytes represent an attractive therapeutic target for the treatment of traumatic brain injury in the glial scar, which inhibits functional repair and recovery if persistent. Many biomaterial systems have been investigated for neural tissue engineering applications, including electrospun nanofibres, which are a favourable biomaterial as they can mimic the fibrous architecture of the extracellular matrix, and are conveniently modified to present biologically relevant cues to aid in regeneration. Here, we synthesised a novel galactose-presenting polymer, poly(L-lysine)–lactobionic acid (PLL–LBA), for use in layer-by-layer (LbL) functionalisation of poly(ε-caprolactone) (PCL) nanofibres, to covalently attach galactose moieties to the nanofibre scaffold surface. We have assessed the use of this novel biomaterial system in vitro and in vivo, and have shown, for the first time, the ability of galactose to maintain an attenuated inflammatory profile of astrocytes in culture, and to increase the survival of neurons after traumatic injury, as compared to control PCL nanofibres. This study highlights the importance of galactose in controlling the astrocytic response, and provides a promising biomaterial system to deliver the essential morphological and biological cues to achieve functional repair after traumatic brain injury.


 Please wait while we load your content...
Please wait while we load your content...