Dual-responsive core-crosslinked polyphosphoester-based nanoparticles for pH/redox-triggered anticancer drug delivery†
Abstract
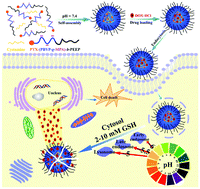
“Intelligent” crosslinked nanoparticles (NPs) provide great advantages in enhancing drug bioavailability and reducing side effects in anticancer therapeutics. In this study, a novel biodegradable polyphosphoester-based functional copolymer prodrug PTX-(PBYP-g-MPA)-b-PEEP was prepared to construct pH/redox dual-responsive core-crosslinked nanoparticles (DOX/CCL NPs), in which paclitaxel (PTX) was conjugated to the polyphosphoester to form an amphiphilic prodrug and doxorubicin (DOX) was encapsulated inside the prodrug NPs. At first, PTX was used as an initiator to polymerize 2-(but-3-yn-1-yloxy)-2-oxo-1,3,2-dioxaphospholane (BYP) and 2-ethoxy-2-oxo-1,3,2-dioxaphospholane (EOP) by one-pot sequential ring-opening polymerization, yielding a biodegradable polymeric prodrug PTX-PBYP-b-PEEP. Subsequently, a radical-mediated thiol–yne “click” reaction was performed between the alkynyl groups on the PBYP segment and the thiol group of 3-mercaptopropionic acid (MPA) to form a functional carboxyl group at the side chain. The potential positively charged DOX·HCl can be physically encapsulated via electrostatic interaction with the carboxyl group and hydrophobic interaction. Afterwards, the DOX/CCL NPs with cleavable disulfide (S–S) linkages can be formed by partial crosslinking through amidation between the pendant carboxyl groups and cystamine. These NPs possess multifunctional characteristics used for in vitro drug release. Notably, a redox-responsive crosslinker, cystamine dihydrochloride, and synergetic non-covalent interactions not only stabilize the nanoparticles, achieve high DOX-loading capacity of drug loading content (DLC, 14.6%) and drug loading efficiency (DLE, 73.1%), but also endow the DOX/CCL NPs with controlled drug release capacity, which is due to the cleavage of S–S bonds in the presence of 10 mM glutathione (GSH) and weakened electrostatic interaction caused by the protonation of carboxyl groups at a lower pH (5.0). Moreover, these pH/redox dual-responsive DOX/CCL NPs can be steadily internalized by HeLa cells, exhibiting high-efficiency cellular proliferation inhibition. This study presents a promising strategy for controlled intracellular drug release in cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...