Exploring the role of molecular chirality in the photo-responsiveness of dipeptide-based gels†
Abstract
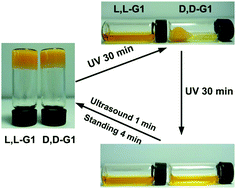
Photo-responsive materials, particularly those based on peptides, hold much promise for numerous potential applications in biomedicine and bionanotechnology. For these switchable materials, one of the key issues is how their photo-response rate can be regulated precisely and reversibly. In this study, we used molecular chirality as a useful tool for modulating the gel–sol response rate of dipeptide-based gels consisting of azobenzene and two dipeptide arms. UV light irradiation triggered the trans-to-cis (E/Z)-isomerization of azobenzene that destroyed the planar structures of gelators and induced gel collapse. During this process, a distinct gel–sol transition speed was identified for the L-gel and D-gel, and remarkable differences were observed in the self-assembly behavior between the L-gelator and the D-gelator. Mechanistic studies revealed that molecular chirality dominated the rearrangement of the flexible dipeptide linkers, which further affected the competitive balance between E/Z-isomerization and gelation forces driven by π–π stacking among adjacent benzenes, thus resulting in the distinct disassembly behaviors of the L-gel and the D-gel. The remarkable difference in the gel–sol transition speed, good reversibility and the underlying competition mechanism suggest that molecular chirality may be an efficient parameter for regulating the performance of photo-responsive gels and devices.


 Please wait while we load your content...
Please wait while we load your content...