In vivo toxicological assessment of electrochemically engineered anodic alumina nanotubes: a study of biodistribution, subcutaneous implantation and intravenous injection†
Abstract
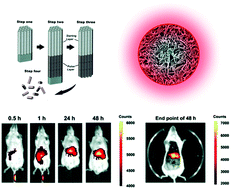
Electrochemically engineered anodic alumina nanotubes (AANTs) have recently shown good in vitro biocompatibility. However, in vivo toxicological and pathological studies are required to clarify the bio-safety of this novel nanomaterial. Herein, we present a pioneering pilot toxicity study on AANTs in immune-competent murine models (Balb/c mice, 8 weeks). AANTs were administered by intravenous (IV) injection and subcutaneous (SC) implantation routes considering the future toxicological implications associated with this nanomaterial for potential biomedical applications. AANTs, 736 nm long and 90 nm in outer diameter, were chosen as a nanomaterial model. We demonstrate that IV injected AANTs do not have any effect on the mortality or body weight of these animal models within 28 days at three different doses (20, 50, 100 mg kg−1). The biodistribution of AANTs characterized by fluorescence imaging and inductively coupled plasma revealed the accumulation of AANTs in the liver and spleen after IV injection. When AANTs were injected intravenously, the highest dose of 100 mg kg−1 caused moderate hepatotoxicity, identified by histopathological analysis. The implantation of AANTs subcutaneously and directly under the skin leads to an inflammatory response, which is a typical foreign body reaction. Taken together, this work provides new insights into the toxicity patterns of new nanomaterials such as AANTs and establishes a rationale for the design of functional AANTs for future biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...