Control of gelation, degradation and physical properties of polyethylene glycol hydrogels through the chemical and physical identity of the crosslinker†
Abstract
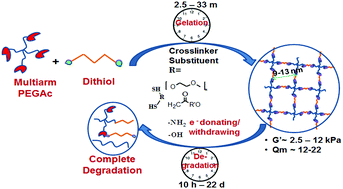
Tuning hydrogel properties through minor modifications of the crosslinker structure is a beneficial approach for hydrogel design that could result in hydrogels with wide range of properties to match a desired application. In this study, we analyzed the relationship between the dithiol crosslinker chemical and physical structure and the resulting properties of polyethylene glycol (PEG) hydrogels formed via Michael-type addition reaction. Specifically, the dithiol crosslinker properties and chemical structure were correlated with gelation time, hydrolytic degradation rate, reaction rate constant, crosslink density and storage modulus of PEG hydrogels. By changing the properties and structure of the crosslinker, hydrogels with controlled degradation ranging from 10 h to 22 d were obtained. It was also established that hydrogel gelation times correlated closely with degradation times. By extensive characterization of the dithiol crosslinker chemical structure and physical properties, we identified two sets of conditions which yielded fast-gelling, fast-degrading hydrogels and slow-gelling, slow-degrading hydrogels. Uniquely, the hydrogel storage moduli could be controlled by the dithiol crosslinker chemical identity independent of the degradation time of the hydrogel or the mesh size.


 Please wait while we load your content...
Please wait while we load your content...