Dual-functional selenium nanoparticles bind to and inhibit amyloid β fiber formation in Alzheimer's disease†
Abstract
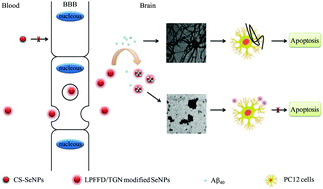
Inhibition of amyloid β (Aβ) aggregation holds considerable promise as a therapeutic strategy for Alzheimer's disease (AD). However, successful inhibition is hard to achieve due to the blood–brain barrier (BBB) and the non-selective distribution of drugs. Herein, two targeting peptides (LPFFD and TGN) were conjugated to selenium nanoparticles (SeNPs). We found that the concentration ratio of LPFFD to TGN taken as 1 : 1 could form the most effective dual-functional SeNPs (L1T1–SeNPs) for inhibiting Aβ aggregation and crossing the BBB. L1T1–SeNPs can cross the BBB and have a strong affinity toward Aβ species, and thus, they can efficiently suppress extracellular Aβ fibrillation by disrupting hydrophobic and electrostatic interactions that are important for Aβ40 nucleation. Also, L1T1–SeNPs can suppress the Aβ40 fiber mediated generation of reactive oxygen species (ROS) and their corresponding neurotoxicity in PC12 cells. In addition, L1T1–SeNPs exert synergistic effects on the inhibition of Aβ aggregation and cross the BBB efficiently. Collectively, these results demonstrate that dual-functional SeNPs might be a valuable targeting system for inhibiting Aβ aggregation.


 Please wait while we load your content...
Please wait while we load your content...