Impact of rotamer diversity on the self-assembly of nearly isostructural molecular semiconductors†
Abstract
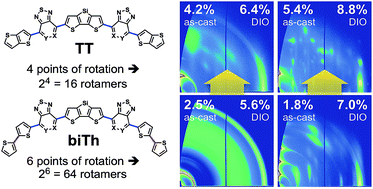
Conformational diversity due to different orientations of structural subunits has a complex impact on morphological disorder of organic semiconductors. Here, we isolate the impact of a specific structural change: replacing bithiophene (biTh) units with thieno[3,2-b]thiophene (TT). We compare four molecules with an alternating donor–acceptor structure (D′–A–D–A–D′) composed of a central, electron-rich dithienosilole (DTS) unit flanked by pyridyl-[2,1,3]thiadiazole (PT) or fluorinated benzo[c][1,2,5]thiadiazole (FBT) and end-capped with bithiophene biTh or TT groups. We find that using TT instead of biTh results in an increased degree of order within films cast directly from solution by influencing the self-assembly tendencies of the different molecules. Unlike switching the acceptor subunit, such as FBT for PT, the TT for biTh structural change has little impact on the electronic structure of these molecular semiconductors. Instead, these morphological effects can be understood within the context of the predicted conformational diversity. TT units limit the number of rotational conformations (rotamers) available within this molecular architecture; low rotamer dispersity facilitates self-assembly into ordered domains. As a practical illustration of this greater drive toward self-assembly, we use the TT-containing molecules as donors in bulk heterojunction solar cells with PC70BM. Devices with TT-containing molecules show improved photovoltaic performance compared to their previously characterized biTh analogs (d-DTS(PTTh2)2 and p-DTS(FBTTh2)2) in both as-cast and optimized conditions, with efficiencies up to 6.4% and 8.8% for PT-TT and FBT-TT, respectively. The TT subunit and, more broadly, the strategy of limiting conformational diversity can be readily applied toward the design of solution-processable organic semiconductors with increased as-cast order.
- This article is part of the themed collection: 2018 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...