Aqueous-solution synthesis of Na3SbS4 solid electrolytes for all-solid-state Na-ion batteries†
Abstract
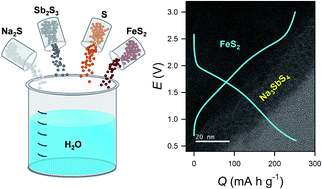
Room-temperature-operable all-solid-state Na-ion batteries (ASNBs) using sulfide Na-ion solid electrolytes (SEs) are promising because of their potential for greater safety, lower cost, and acceptable performance. Despite extensive developments in the area of sulfide Na-ion SEs, their poor chemical stability and prospects for wet-chemical synthesis have been overlooked to date. Herein, the scalable synthesis of Na3SbS4via aqueous-solution routes using precursors of Na2S, Sb2S3, and elemental sulfur for ASNBs is described. With no concerns about the evolution of toxic H2S gas, the aqueous-solution-synthesized Na3SbS4 exhibits high ionic conductivities (0.1–0.2 mS cm−1 at 25 °C). Importantly, the homogeneity of the aqueous solutions enables the creation of uniform Na3SbS4 coatings on FeS2. Fe2S/Na–Sn ASNBs, employing the aqueous-solution-synthesized Na3SbS4 and the Na3SbS4-coated FeS2 for the SE layer and positive electrode, respectively, demonstrate a high charge capacity of 256 or 346 mA h g−1 with good reversibility at 30 °C, highlighting their potential for practical applications.


 Please wait while we load your content...
Please wait while we load your content...