A benzyltetramethylimidazolium-based membrane with exceptional alkaline stability in fuel cells: role of its structure in alkaline stability
Abstract
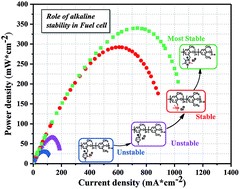
A highly alkaline stable anion exchange membrane is a critical component in alkaline polyelectrolyte fuel cells (APEFCs). However, the commonly employed cationic moieties in the membrane show limited alkaline stability, thereby barely enduring harsh fuel cell operating conditions. In this study, we synthesized a series of imidazolium-type anion-exchange membranes, in which the substituents were attached at different positions of the imdazolium ring to systematically investigate their impact on the overall alkaline stability of the cationic moieties. 1,2,4,5-Tetramethylimidazolium-functionalized anion-exchange membrane has highest alkaline stability, and it retains 97% alkaline stability after 7 days in a 1 M NaOD/D2O/CD3OD solution at 60 °C. Compared to the recently reported imidazolium-type membranes, the resultant membrane exhibits unprecedented fuel cell performance to date, reaching an exceptional excellent peak power density of 340 mW cm−2 at 80 °C.


 Please wait while we load your content...
Please wait while we load your content...