Investigation of chloride ion adsorption onto Ti2C MXene monolayers by first-principles calculations†
Abstract
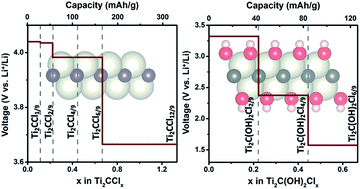
Chloride ion adsorption on Ti2C monolayers was theoretically investigated. Electrochemical parameters, including specific capacity, chloride ion (Cl−) diffusion barrier, and discharge voltage profile, were studied via first-principles calculations. The most favorable Cl− adsorption configuration was identified using a partial particle swarm optimization algorithm and the results showed that Cl− adsorption onto Ti2C monolayers achieved a large theoretical capacity (331 mA h g−1), high working voltage (4.0–3.5 V), and low diffusion barrier (0.22 eV). This resulted in excellent rate capability and a large specific energy of 1269 W h kg−1 at the material level. The effects of terminal O, F, and OH groups on Cl− adsorption onto Ti2C monolayer were also studied, which showed that Cl− could not be adsorbed onto O and F terminated Ti2C monolayers. In comparison, Cl− adsorption onto OH terminated Ti2C was allowed but generated a smaller specific capacity (126 mA h g−1) and lower working voltage (3.5–1.5 V) than a bare Ti2C monolayer.


 Please wait while we load your content...
Please wait while we load your content...