Colloidal synthesis of monodisperse trimetallic IrNiFe nanoparticles as highly active bifunctional electrocatalysts for acidic overall water splitting†
Abstract
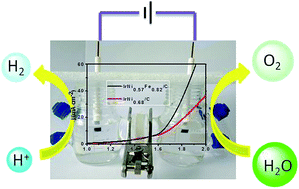
The search for high-performance bifunctional electrocatalysts for overall water splitting under acidic conditions is highly desirable for the development of polymer electrolyte membrane (PEM) electrolyzers, whereas, there are still many challenges to fabricate satisfactory electrocatalysts that could exhibit excellent activity with long-term stability. Herein, we report the colloidal synthesis of monodisperse trimetallic IrNiFe nanoparticles (NPs) with an average diameter of 2.2 nm. By taking advantage of ultrasmall monodisperse NPs with narrow size distribution and the strong synergistic electronic effect between Ir, Ni and Fe, the resultant IrNi0.57Fe0.82 NPs exhibit good activity and excellent durability for both the HER and OER in acidic electrolyte. To reach a current density of 10 mA cm−2, the overpotential of the HER and OER in 0.5 M HClO4 is 24 and 284 mV, respectively. Furthermore, the practical application of IrNi0.57Fe0.82 as a bifunctional catalyst for acidic overall water splitting yields a current density of 10 mA cm−2 at 1.64 V with long-term stability.


 Please wait while we load your content...
Please wait while we load your content...