Vanadyl-type defects in Tavorite-like NaVPO4F: from the average long range structure to local environments†
Abstract
Tavorite-type compositions offer rich crystal chemistry for positive electrodes in rechargeable batteries, among which LiVIIIPO4F has the highest theoretical energy density (i.e. 655 Wh kg−1). In this article, we report for the first time the synthesis of the related Na-based phase crystallizing in the Tavorite-like structure. Its in-depth structural and electronic characterization was conducted by a combination of several techniques, spanning electron and X-ray powder diffraction as well as infrared and X-ray absorption spectroscopy. The magnetic susceptibility measurement reveals an average oxidation state for vanadium slightly higher than V3+. This slight oxidation is supported by infrared and X-ray absorption spectroscopies which highlight the presence of V4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vanadyl-type defects leading to an approximated NaVIII0.85(VIVO)0.15(PO4)F0.85 composition. In this material, the profile of the diffraction lines is governed by a strong strain anisotropic broadening arising from the competitive formation between the ionic V3+–F and the covalent V4+
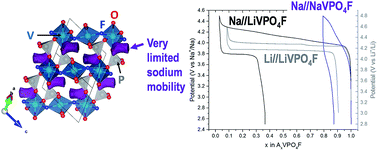
O vanadyl-type defects leading to an approximated NaVIII0.85(VIVO)0.15(PO4)F0.85 composition. In this material, the profile of the diffraction lines is governed by a strong strain anisotropic broadening arising from the competitive formation between the ionic V3+–F and the covalent V4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. This material shows a limited extraction of sodium, close to 15% of the theoretical capacity. Indeed, its electrochemical properties are strongly inhibited by the intrinsic low sodium mobility in the Tavorite framework.
O bonds. This material shows a limited extraction of sodium, close to 15% of the theoretical capacity. Indeed, its electrochemical properties are strongly inhibited by the intrinsic low sodium mobility in the Tavorite framework.


 Please wait while we load your content...
Please wait while we load your content...