Amorphous Co–Fe–P nanospheres for efficient water oxidation†
Abstract
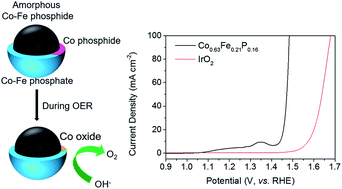
Transition metal phosphides are often studied as hydrogen evolution reaction cathode catalysts, but scarcely as oxygen evolution reaction (OER) anode catalysts for water oxidation. Herein, we report a new amorphous Co0.63Fe0.21P0.16 OER catalyst, which was prepared through a one-step solvothermal process. This catalyst exhibits extraordinary OER catalytic activity in alkaline media and is superior to the state of the art IrO2 in 1.0 M KOH, capable of yielding a current density of 10 mA cm−2 at an overpotential of only 217 mV. This newly achieved high activity outperforms most reported transition metal phosphide catalysts. During the OER, the surface layers of the Co0.63Fe0.21P0.16 nanospheres (200–500 nm) are oxidized, which show the typical Tafel slope of oxides around 40 mV dec−1. The in situ formation of surface Co oxides in the catalysts during the OER along with an optimal doping of Fe are found to be crucial for their remarkable activity. In particular, the metal-rich amorphous phosphide cores, i.e., substrates, probably contribute to OER performance of the catalysts due to their high electrical conductivity. This new type of surface oxidized amorphous transition metal phosphide would provide a new pathway for the design of high-performance OER electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...