Encapsulating nanoscale zero-valent iron with a soluble Mg(OH)2 shell for improved mobility and controlled reactivity release†
Abstract
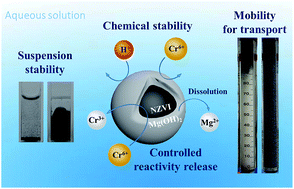
Nanoscale zero-valent iron (NZVI) is a highly reactive material for decontamination but it often suffers from problems such as agglomeration, aqueous corrosion, and poor mobility for field applications. In this study, a new modification technique was developed by coating the NZVI surface with a soluble Mg(OH)2 shell to form Mg(OH)2-coated NZVI (NZVI@Mg(OH)2) nanoparticles. The Mg(OH)2 shell significantly reduced the magnetic attraction between NZVI particles, preventing particle aggregation. Consequently, NZVI@Mg(OH)2 with an appropriate coating ratio (Mg/Fe, wt%) had an increased stability in suspension and considerably improved mobility in saturated porous media. According to the filtration tests, the Mg(OH)2 shell effectively decreased the attachment, blocking, and straining effects during the transport of NZVI particles through a sand column. With the Mg(OH)2 shell, the reactivity of NZVI was well preserved during the NZVI storage and transport. Encapsulation with 100 wt% Mg(OH)2 could protect the NZVI core from aqueous corrosion such as oxidation by H+ ions. When NZVI@Mg(OH)2 is used in field applications, the NZVI reactivity would be progressively released and fully recovered with the dissolution of the Mg(OH)2 shell in the background water. The test results showed that after the dissolution of the Mg(OH)2 shell, the NZVI exhibited a similar reactivity and capacity to bare NZVI for the reduction of Cr(VI). Overall, the novel Mg(OH)2 coating technique not only provides NZVI with a high mobility for transport and delivery but also protects the NZVI core from rapid corrosion and enables a controlled release of NZVI reactivity for environmental remediation.
- This article is part of the themed collections: International Year of the Periodic Table: Applications for magnetic materials and 2018 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...