Hierarchical Ni/NiTiO3 derived from NiTi LDHs: a bifunctional electrocatalyst for overall water splitting†
Abstract
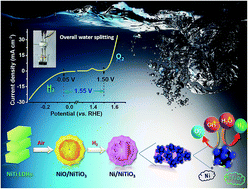
Developing economical and stable bifunctional electrocatalysts for overall water splitting is of enormous importance for sustainable energy systems. Here, Ni/NiTiO3 with a villiform structure assembled by nanosheets is presented as an efficient bifunctional electrocatalyst. The Ni/NiTiO3 with a molar ratio of 15 : 1 (Ni : Ti) manifests remarkable catalytic performance for both the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) in alkaline solutions, with onset overpotentials of 270 mV for the OER and ∼50 mV for the HER. Large amounts of Ni nanoparticles inset in the NiTiO3 nanosheets possess a high specific surface of 28.4 m2 g−1 which is far greater than that of bare Ni (7.7 m2 g−1). The results of long-term OER operation confirm that the introduction of Ti4+ is favorable for enhanced stability. When the catalyst is employed as both the cathode and anode for overall water splitting, it displays an onset potential of 1.55 V in 1 M KOH solution which can rival that of the integrated performance of Pt/C and RuO2. Hence, our Ni/NiTiO3 electrocatalysts have enormous potential for realistic large-scale water splitting.


 Please wait while we load your content...
Please wait while we load your content...