Intermetallic Pd3Pb nanowire networks boost ethanol oxidation and oxygen reduction reactions with significantly improved methanol tolerance†
Abstract
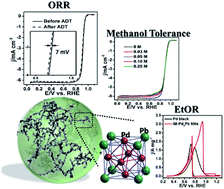
Intermetallic nanocrystals are currently receiving extensive attention due to their well-defined crystal structures, highly ordered atomic distribution and superior structural stability that endow them with optimized catalytic activities, stabilities and high selectivity for use as electrocatalysts for fuel cells. Here, for the first time, we reported the facile synthesis of intermetallic Pd3Pb nanowire networks (IM-Pd3Pb NNs) with a one-step wet-chemical strategy at a relatively low temperature (i.e. 170 °C) in 1 h. The as-prepared IM-Pd3Pb NNs exhibited superior bifunctional catalytic performances toward the oxygen reduction reaction (ORR) and the ethanol oxidation reaction (EtOR) compared to commercial Pt/C and Pd black, respectively. Significantly, IM-Pd3Pb NNs also showed excellent methanol- and CO-tolerant ability as ORR cathode and EtOR anode electrocatalysts, respectively. The electrochemically active surface area and mass activity of IM-Pd3Pb NNs are about 3.4 times and 2 times higher than those of Pd black toward the EtOR, respectively. As the Pt-free bifunctional electrocatalysts, 3D IM-Pd3Pb architectures with exceptional catalytic performances hold great promise in various applications such as energy conversion and storage devices, sensors, electronics, optics and so on.


 Please wait while we load your content...
Please wait while we load your content...