Galvanic-replacement mediated synthesis of copper–nickel nitrides as electrocatalyst for hydrogen evolution reaction†
Abstract
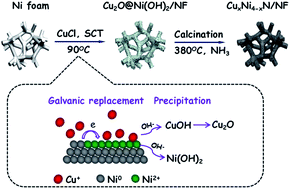
Electrochemical water splitting is considered to be one of the most promising strategies to produce hydrogen to ease the energy crisis. In this study, we demonstrate a galvanic-replacement mediated synthesis of copper-nickel bimetallic nitrides on partially sacrificial nickel foams (CuxNi4−xN/NF) as the direct catalytic electrode for hydrogen evolution reaction (HER). The Ni foam serves as not only the cathodic electrode substrate, but also the only Ni precursor for the CuxNi4−xN catalyst, which can be galvanically replaced by Cu(I) ion. The obtained CuxNi4−xN/NF exhibits an excellent electrocatalytic performance towards the HER in both acidic and alkaline media with a very low overpotential of ∼110 mV at a current density of 100 mA cm−2 in the 0.5 M H2SO4 and 1 M KOH solutions. The electrode presents a good long-term working stability, particularly reflecting in more than 65 h of consistent galvanostatic electrolysis in 0.5 M H2SO4. The CuNi bimetallic nitrides are intrinsically metallic, allowing for an enhanced charge transport and an excellent electrical conductivity. Combining the experimental result and the theoretical calculation further reveals that the electrocatalytic active sites primarily originate from nickel species.


 Please wait while we load your content...
Please wait while we load your content...