3D hollow porous carbon microspheres derived from Mn-MOFs and their electrochemical behavior for sodium storage
Abstract
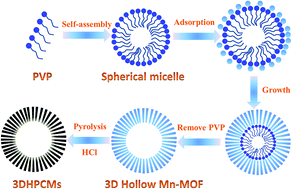
Extensive efforts have been put into developing new materials with a 3D hollow porous spherical structure for increasing their applications in energy storage. In this work, 3D hollow porous carbon microspheres (3DHPCMs) are firstly prepared by the carbonization and post acid-treatment of 3D hollow microspherical Mn-MOFs (3DMn-MOFs), showing a high surface area of 788.2 m2 g−1 and a diameter of about 2 μm. Importantly, this is the first time that the conversion from 1D nanorods to 3D hollow spheres of Mn-MOFs through regulating the amount of poly(vinylpyrrolidone) (PVP) has been realized. Besides, the sodium storage behavior of 3D hollow porous carbon microspheres is also firstly studied. When utilized as anodes for sodium ion batteries (SIBs), the 3DHPCMs deliver excellent electrochemical storage performances with a high specific capacity of 313.8 mA h g−1 at a current density of 100 mA g−1. Impressively, a high discharge specific capacity of 112.5 mA h g−1 is obtained at 5 A g−1. The outstanding electrochemical performances can be attributed to the 3D hollow porous microsphere structure, which can enhance the mechanical stability, buffer the volume expansion, and accelerate the transport of Na+ and electrons. This work provides a new route for the development of materials with a 3D hollow spherical structure.


 Please wait while we load your content...
Please wait while we load your content...