Mapping a stable solvent structure landscape for aprotic Li–air battery organic electrolytes†
Abstract
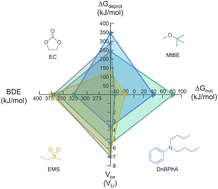
Electrolyte instability is one of the greatest impediments that must be overcome for the practical development of rechargeable aprotic Li–air batteries. In this work, we establish a comprehensive framework for evaluation of the stability of potential organic electrolytes for aprotic Li–air batteries that is based on four key descriptors: Bond dissociation energy, deprotonation free energy (i.e., Acidity), Nucleophilic substitution free energy, and Electrochemical oxidation/reduction. These parameters were calculated for several classes of organic compounds. The chemical stability of the molecules was studied experimentally under conditions designed to mimic the aprotic Li–air battery environment (heating in the presence of excess KO2 and Li2O2). In general, the calculated and experimental data agreed well for alkanes, alkenes, ethers, aromatics, carbonates, and S-containing and N-containing compounds. Using this dataset, we identified functional groups and other structural features of organic molecules that may be suitable for aprotic Li–air battery electrolyte design.
- This article is part of the themed collection: Celebrating Excellence in Research: Women of Materials Science


 Please wait while we load your content...
Please wait while we load your content...