Barium disilicide as a promising thin-film photovoltaic absorber: structural, electronic, and defect properties†
Abstract
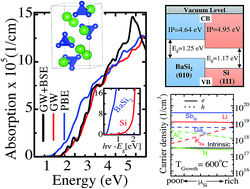
Barium disilicide (BaSi2), composed of abundant and inexpensive elements, is a potential absorber material for thin-film solar cells. In this study, density-functional theory calculations show that BaSi2 belongs to a Zintl phase with a mixed character of covalent units of tetrahedral Si4 with an ionic nature described by (2Ba2+)(Si4)4−. The molecular orbital diagram is elucidated based on the electronic structures, suggesting that the charge transfer transition from Si p to Ba d greatly enhances optical absorption. A large photoabsorption coefficient is confirmed using advanced excited state calculations that include excitonic effects. The ionization potential of BaSi2 is smaller than that of silicon or germanium, suggesting that the band edge positions are suitable for p-type conductivity and type II heterojunctions for BaSi2/Si or BaSi2/Ge. The chemical potential window for the stable growth of a stoichiometric BaSi2 film is very narrow, and the stability of native defects is investigated under realistic growth conditions. Si vacancy, Ba substituted for Si antisite, and Si interstitial defects are predominant but do not cause the generation of a significant number of carriers. The calculated Fermi level is pinned in the middle of the band gap for the entire silicon chemical potential range and a wide growth temperature range, indicating the feasibility of bipolar doping, which is advantageous for fabricating p–n junctions.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...