Enhancing metallic lithium battery performance by tuning the electrolyte solution structure†
Abstract
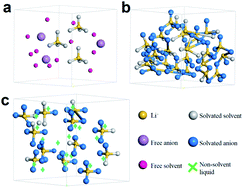
A concentrated electrolyte shows a series of outstanding properties including improved lithium plating/stripping performance and suppressed shuttle effect for Li–S batteries owing to its special solvation sheath of Li+. However, its high viscosity, low conductivity and poor wettability have to be addressed before its wide application, which are caused by its solution structure. Here, based on tuning the solution structure of the electrolyte while maintaining the solvation sheath of Li+, a non-solvent liquid is introduced into the concentrated electrolyte (CE), forming a novel pseudo-concentrated electrolyte (PCE). Not only does the solvation structure of Li+ in the PCE not change because lithium salt can't be dissolved in the non-solvent liquid, but it is also strengthened owing to the anti-solvation effect. Therefore, a PCE with high conductivity and low viscosity is obtained. The PCE further displays improved merits of the concentrated electrolyte including improved lithium metal plating/stripping performance, improved oxidation stability and inhibited dissolution of lithium polysulfides. Moreover, based on the anti-solvation effect, a strengthened pseudo-concentrated electrolyte (SPCE) with a lower concentration of lithium salt is obtained. It displays super properties including high conductivity, low viscosity, and low cost and further suppresses the shuttle effect, indicating promising applications which are not limited to Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...