Improved performance through tight coupling of redox cycles of sulfur and 2,6-polyanthraquinone in lithium–sulfur batteries†
Abstract
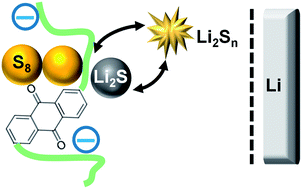
Lithium–sulfur (Li–S) batteries offer high theoretical capacity and energy density; however, complex nanoengineered cathodes have been required to suppress the unwanted polysulfide shuttling. The textural complexity of such electrodes hinders detailed understanding of their function, impeding the development of new materials. In this report, the redox-active polymer 2,6-polyanthraquinone (PAQ) was incorporated into the cathode. The presence of this polymer improves capacity retention in galvanostatic cycling and inhibits Li corrosion and S deposition. We show that redox reactions of this polymer are strongly coupled to the S redox cycle and hypothesize that the observed improvements in the performance originate from the electrocatalytic inhibition of polysulfide shuttling in this system.


 Please wait while we load your content...
Please wait while we load your content...