Exceptionally high sodium-ion battery cathode capacity based on doped ammonium vanadium oxide and a full cell SIB prototype study†
Abstract
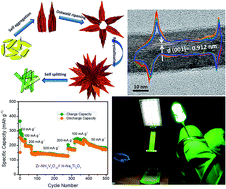
Sodium-ion battery technology, the existing electrodes, and electrolytes are still in the early stage of development, and more intense research is necessary before moving to mass production and application. High capacity and rate capable electrodes are a mandate, and very few combinations of cathode, anode, and electrolyte have been reported with an excellent full cell performance with a long cycle life. Herein, we report for the first time an ultrahigh specific sodium discharge capacity (∼342 mA h g−1 at 0.1 A g−1 current rate) and excellent electrochemical performance of a doped ammonium vanadium oxide (NVO) cathode. The cathode performance exhibited >99% capacity retention after 50 cycles at a very high current rate of 2 A g−1. Furthermore, the present report demonstrates the full cell performance with a doped ammonium vanadium oxide (NVO) cathode against a hydrogenated sodium titanium oxide (NTO) anode. The full cell is capable of retaining 94% capacity after 400 cycles and also demonstrated its potential application in an LED-based table lamp. The present study shows a way to produce a rechargeable sodium-ion battery full cell and can be deciphered to facilitate large-scale renewable energy (RE) storage.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...