Highly active two dimensional α-MoO3−x for the electrocatalytic hydrogen evolution reaction†
Abstract
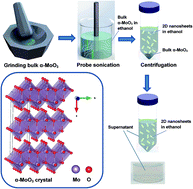
The development of earth-abundant electrocatalysts for hydrogen evolution, with high activity and stability, is of great interest in the field of clean energy. The highly tunable chemical and physical properties of earth-abundant molybdenum oxides make them versatile for their incorporation into electrochemical and catalytic systems. Due to the layered crystal arrangement of orthorhombic α-MoO3, this material can be exfoliated into two dimensional (2D) nanosheets, featuring a large surface area. Variations in the oxidation states of molybdenum facilitate the crystal structure, morphology and oxygen vacancy tuning, making these oxide compounds suitable for electrochemical activities. Here, oxygen deficient 2D α-MoO3−x nanosheets (x = 0.045) are successfully synthesised, using a liquid phase exfoliation method, which display superior activity for the electrocatalytic hydrogen evolution reaction (HER) with a low overpotential and fast electron transfer. In alkaline media, the 2D compound exhibits an overpotential value of 142 mV at the standard current density of 10 mA cm−2 with excellent stability. Here, the 2D morphology, structural defects and oxygen vacancies in the planar construction of molybdenum oxide nanosheets significantly increase the active sites of the catalyst, which act as key factors to promote the HER performance. This work presents 2D α-MoO3−x nanosheets as strong candidates for the HER.


 Please wait while we load your content...
Please wait while we load your content...