Ambient temperature aqueous synthesis of ultrasmall copper doped ceria nanocrystals for the water gas shift and carbon monoxide oxidation reactions†
Abstract
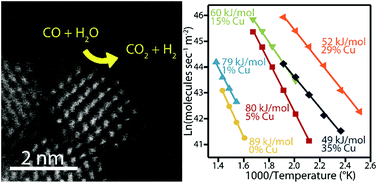
Ceria substitutionally doped with copper is a promising heterogeneous catalyst for a range of oxidation reactions. Herein we describe the aqueous phase, scalable, and direct precipitation of CuxCe1−xO2−δ (x = 0–0.35) solid solution oxide nanocrystals at room temperature without the need for calcination at elevated temperatures. This direct precipitation of the crystalline oxide is enabled through ligand exchange prior to pH adjustment to prevent the precipitation of the hydroxide phase. By producing particles at room temperature, dopant exsolution and particle growth by sintering can be minimized and/or controlled. Using our methodology, copper dopant concentrations of up to 35 mol% could be produced in 1.7 nm diameter ceria nanocrystals. The resulting materials showed high catalytic activity towards both the water gas shift reaction (WGS) and CO oxidation, with improved performance following the trend of increasing copper content. In comparison to pure ceria nanocrystals, the WGS activation energy decreased from 89.0 to 49.2 kJ mol−1 and the CO oxidation light-off temperature decreased from 262 to 159 °C at a space velocity of 25 000 h−1 upon doping with 35 mol% copper.


 Please wait while we load your content...
Please wait while we load your content...