Egg-shell structured LiCoO2 by Cu2+ substitution to Li+ sites via facile stirring in an aqueous copper(ii) nitrate solution†
Abstract
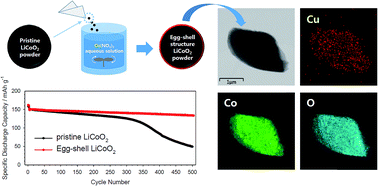
For practical, high-energy lithium ion batteries, we introduce an egg-shell structured LiCoO2, enabling a credible performance with a high cut-off potential of 4.4 V, simply prepared by only stirring in 0.5 mM Cu(NO3)2 aqueous solution at room temperature without costly heat treatment. Through this, a very robust structure in which the Li+ ions in the LiCoO2 structure were substituted with Cu2+ is selectively synthesized on the surface of active material particles. The egg-shell structured LiCoO2 presents excellent cyclability and high coulombic efficiency even in a high potential range of 4.4 V (vs. Li/Li+) to allow a high specific capacity. Additionally, the surface-modified LiCoO2 reaches a high capacity at a fast discharge rate of 20C even after a high temperature cycling sequence. The intended surface modification is also carefully investigated by systematic analyses of STEM, SEM and XPS. Finally, the electrochemical performance of a graphite/surface-modified LiCoO2 full-cell exhibits an excellent capacity retention of nearly 90% over 1000 cycles. It is concluded that the surface modification method in which the LiCoO2 powder is added to a low-concentration aqueous solution of copper nitrate and stirred at room temperature is effective in improving the cycle life of LiCoO2 at high potentials.


 Please wait while we load your content...
Please wait while we load your content...