Hydrogen absorption in 1 nm Pd clusters confined in MIL-101(Cr)†
Abstract
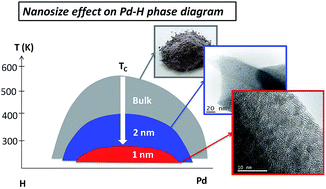
We report here the unprecedented modification of the hydrogen absorption/desorption properties of 1 nm Pd clusters relative to the bulk and nanoparticles down to 2–3 nm. These metal clusters have been synthesized by a facile double solvent impregnation method. They contain on average 33 atoms and are confined/stabilized into a metal-organic-framework with different metal loadings (5–20 wt%). This is the first time, to the best of our knowledge, that 1 nm Pd clusters are effectively confined into a MOF for high metal loadings. Such ultra-small nanoparticles are crystalline with the archetypical fcc structure of the bulk metal, as confirmed by both HR-TEM and in situ EXAFS. Hydrogen absorption/desorption properties of 1 nm Pd clusters have been characterized by both laboratory and synchrotron facilities. Under ambient conditions, 1 nm Pd clusters absorb hydrogen forming solid solutions instead of a hydride phase, as usually encountered for the bulk and Pd nanoparticles down to 2–3 nm. This can be understood by a decrease of the critical temperature of the two-phase region in the Pd–H phase diagram below room temperature. Moreover, the activation energy of hydrogen desorption from Pd clusters strongly decreases relative to bulk Pd. This suggests a change in the rate limiting step from surface recombination or β → α phase transformation usually encountered in bulk Pd to hydrogen diffusion into α and β phases in 1 nm clusters.


 Please wait while we load your content...
Please wait while we load your content...