Well-dispersed molybdenum nitrides on a nitrogen-doped carbon matrix for highly efficient hydrogen evolution in alkaline media†
Abstract
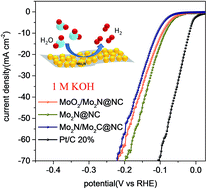
Well-dispersed and highly efficient molybdenum nitrides on a nitrogen-doped carbon matrix (Mo2N@NC) are reported as a new and active electrocatalyst for hydrogen evolution in alkaline electrolyte. The key point in fabricating this catalyst is using HNO3-treated melamine, other than pristine melamine, as carbon and nitrogen pools to realize the complete nitridation of MoO3 to Mo2N nanoparticles of 4 nm size and at the same time to generate a nitrogen-doped carbon matrix to support these nanoparticles by thermolysis under an Ar atmosphere. The as-prepared Mo2N@NC exhibits excellent HER activity in 1 M KOH with a pretty low overpotential of 85 mV to achieve a current density of 10 mA cm−2 and a small Tafel slope of 54 mV dec−1, which is among the best for Mo-based compounds and better than those of most non-noble metal electrocatalysts to date in alkaline media. It is also much better than those of the incomplete nitride compounds (MoO2/Mo2N@NC) and the mixture of nitrides and carbides (Mo2N/Mo2C@NC) fabricated under similar conditions, from aspects of overpotential, Tafel slope, exchange current density, conductivity, active surface area and turnover frequencies. Therefore, Mo2N represents a new kind of very active HER catalyst in alkaline electrolyte. Also, this work provides an effective method to fabricate metal nitrides/carbon composites for other applications.


 Please wait while we load your content...
Please wait while we load your content...