Insights into the increased degradation rate of CH3NH3PbI3 solar cells in combined water and O2 environments†
Abstract
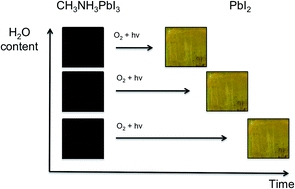
Halide perovskites offer low cost and high efficiency solar cell materials but serious issues related to air and moisture stability remain. In this study we show, using UV-vis, fluorescence and time of flight secondary ion mass spectrometry (ToF-SIMS) techniques, that the degradation of methylammonium lead iodide solar cells is significantly accelerated when both air and moisture are present in comparison to when just air or moisture is present alone. Using ab initio computational techniques we identify the thermodynamic driving force for the enhanced reactivity and highlight the regions of the photoexcited material that are the most likely reaction centres. We suggest that water catalyses the reaction by stabilising the reactive superoxide species, enabling them to react with the methylammonium cation.
- This article is part of the themed collections: International Year of the Periodic Table : From Pb and Sn Perovskites to the Next Generation and In memory of Paul O’Brien


 Please wait while we load your content...
Please wait while we load your content...