Chelating functional group attached to carbon nanotubes prepared for performance enhancement of vanadium redox flow battery†
Abstract
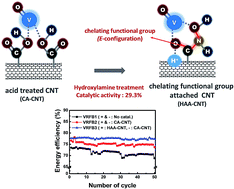
A hydroxamic acid functionalized carbon nanotube (HAA-CNT) catalyst is prepared to determine its effect on VO2+/VO2+ and V2+/V3+ redox reactions and on the performance of a vanadium redox flow battery (VRFB), while its catalytic activity and VRFB performance are compared with those of pure CNTs and carboxylic acid functionalized CNT (CA-CNT) catalysts. According to the cyclic voltammetry measurements, HAA-CNT shows a better catalytic activity and reaction reversibility for vanadium redox reactions than CNT and CA-CNT because of the chelation ability of the HAA included in the HAA-CNT. With the role of chelating agent, the HAA group interacts well with vanadium ions, promoting their redox reactions. In particular, the HAA-CNT is more effective for the VO2+/VO2+ reaction than the V2+/V3+ reaction because of the larger number of oxygen atoms considered as active sites for the VO2+/VO2+ reaction. The role promoting VO2+/VO2+ reaction is confirmed by measuring charge transfer resistance efficiencies of a single cell VRFB. The chemical structure of the HAA-CNT is determined using X-ray photoelectron spectroscopy (XPS). Eventually, when the HAA-CNT is used as a positive electrode for the VO2+/VO2+ reaction, the performance of single cell VRFB is best.


 Please wait while we load your content...
Please wait while we load your content...