Constructing noble-metal-free Z-scheme photocatalytic overall water splitting systems using MoS2 nanosheet modified CdS as a H2 evolution photocatalyst†
Abstract
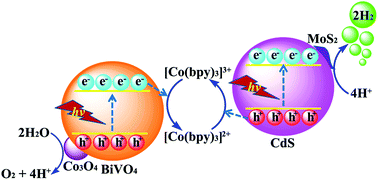
Photocatalytic overall splitting of H2O into H2 and O2 using Z-scheme systems is a promising method for solar hydrogen generation. Most currently designed Z-scheme systems are usually based on precious metal cocatalyst modified semiconductor photocatalysts, thus exploiting noble-metal-free cocatalysts to replace precious metals is of huge interest. Herein, a noble-metal-free Z-scheme photocatalytic overall water splitting system was constructed by using MoS2/CdS as the H2 evolution photocatalyst, Co3O4/BiVO4 as the oxygen evolution photocatalyst and a [Co(bpy)3]3+/[Co(bpy)3]2+ redox couple as an electron mediator under visible light irradiation. It is found that the H2 and O2 evolution rates are approximate to an ideal ratio of 2 : 1, and the highest H2 and O2 evolution rates under visible light irradiation (λ > 420 nm) are found to be 14.5 and 7.1 μmol h−1, respectively, with an excellent stability exceeding 12 h. The apparent quantum yield reaches up to 1.04% at wavelength λ = 420 nm even without any noble metal. The excellent stability and efficiency of this designed Z-scheme system in neutral water demonstrate its potential use for solar hydrogen generation. This work provides a noble-metal-free approach to construct the Z-scheme photocatalytic overall water splitting system.


 Please wait while we load your content...
Please wait while we load your content...