Maximizing the utilization of Fe–NxC/CNx centres for an air-cathode material and practical demonstration of metal–air batteries†
Abstract
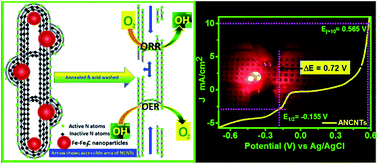
Noble-metal free carbon-based electrocatalysts for oxygen reduction/evolution reaction (ORR/OER) in an alkaline medium have drawn significant attention in the search of suitable/potential replacement of commercial expensive Pt/Ru/Ir-based electrocatalysts. In this regard, nitrogen-doped carbon nanotubes (NCNTs) have emerged as potential candidates for ORR/OER and they emulate Pt/Ru-based catalysts. However, the unexposed/unused active sites rendered by N-doping, responsible for bamboo-shaped compartment formation, in NCNTs are hardly accessible to the reactant species and thus barely contribute to ORR/OER. To maximize the utilization of the active centres, Fe–Fe3C nanoparticles entrapped in the graphitic layers and hollow structures of NCNTs are fabricated by pyrolyzing melamine with excess of ferrocene. Annealing at 375 °C in an oxygen environment removes the protective graphitic layers around Fe–Fe3C, and acid-treatment provides an opening to the bamboo compartments of NCNTs (ANCNTs); this offers maximum accessibility of the active sites via the creation of more edges and roughness that enhances the ORR/OER activity. Interestingly, more positive ORR onset and half-wave potentials with excellent accelerated stability (∼98% current retention) and fuel tolerance along with lower onset and Ej=10(OER) potential with high OER current density as compared to those of commercial state-of-the-art electrocatalyst suggest the superior bifunctional behaviour of ANCNTs. The poisoning study with NaSCN on ANCNTs shows the direct involvement of Fe-based centres in oxygen electrochemistry. The overall oxygen electroactivity ΔE = Ej(OER)=10 − E1/2(ORR) = 0.72 V for ANCNTs is much lower than that of the commercial and recently reported various state-of-the-art bifunctional catalysts. Finally, a prototype battery arrangement using NCNTs as a cathode electrode for powering a light-emitting diode has been demonstrated. Overall, NCNTs can serve as non-precious electrocatalysts for electrochemical energy devices.


 Please wait while we load your content...
Please wait while we load your content...