Hydrogen evolution reaction activity of nickel phosphide is highly sensitive to electrolyte pH†
Abstract
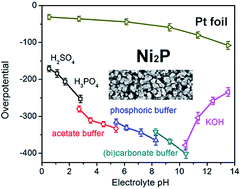
The nickel phosphide (Ni2P) family of materials have become a hot subject in hydrogen evolution reaction (HER) electrocatalyst research. Various studies have reported their high activity, high stability, and high faradaic efficiency. To date, there have been no systematic studies regarding the influence of pH on the HER performance of Ni2P. Here we show that the pH of electrolytes can strongly influence the HER activity of Ni2P electrocatalysts. Tests in 19 electrolytes with pH ranging from 0.52 to 13.53 show that Ni2P is much more active in strongly acidic and basic electrolytes. With the increase of pH, the lower H+ concentration reduces the formation of adsorbed H atoms in the Volmer reaction, resulting in poorer activities. However, the high activity observed in the strongly basic electrolytes is not the intrinsic property of Ni2P. We found that Ni oxides/hydroxides are formed in strongly basic electrolytes under applied potentials, resulting in improved activities. Furthermore, the specific activity based on the electrochemically active surface area of recently reported Ni2P catalysts is not high and requires significant improvements for practical applications.


 Please wait while we load your content...
Please wait while we load your content...