Hydrogen and methane storage and release by MoS2 nanotubes for energy storage†
Abstract
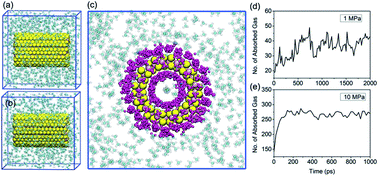
Using molecular dynamics simulations, we investigate the performance of molybdenum disulfide nanotubes (MoS2 NTs) as a medium for energy gas storage (hydrogen and methane). Two representative MoS2 NTs, (12, 12) and (6, 6), are considered in our present study. MoS2 NTs are found to effectively attract hydrogen and methane molecules through their surface and interior. Under storage conditions (175 K and 10 MPa), the storage capacity of methane in MoS2 NTs can reach 7–8 wt%, depending on the diameter of MoS2 NTs. For hydrogen, the storage capacity is around 0.7–0.9 wt%. The gas weight percentage is linearly dependent on the environmental pressure from 10 MPa to 1 MPa at constant temperature. Meanwhile, adsorption demonstrates an exponential relationship with the system temperature from 175 K to 425 K when pressure is maintained constant. Dynamic pressure simulations reveal that up to 80–90% of the stored gases can be spontaneously released as the pressure decreases from 10 MPa to 1 MPa, with methane slightly more efficient than hydrogen, indicating high cyclic storage performance. Our present theoretical findings shed new light on the utilization of MoS2 nanomaterials as a novel gas storage platform and we hope our results will promote future experimental efforts.


 Please wait while we load your content...
Please wait while we load your content...