Physisorption-induced structural change directing carbon monoxide chemisorption and nitric oxide coordination on hemilabile porous metal organic framework NaNi3(OH)(SIP)2(H2O)5·H2O (SIP = 5-sulfoisophthalate)†
Abstract
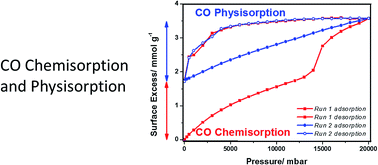
Structural changes occur during the thermal activation of NaNi3(OH)(SIP)2(H2O)5·H2O and NaCo3(OH)(SIP)2(H2O)5·H2O to form porous framework materials. Activation of NaNi3(OH)(SIP)2(H2O)5·H2O at 400 K gave NaNi3(OH)(SIP)2(H2O)2 and 513 K gave NaNi3(OH)(SIP)2. CO adsorption/desorption on NaNi3(OH)(SIP)2(H2O)2 at 348 K and 20 bar was hysteretic, but all CO was desorbed in vacuum. NaNi3(OH)(SIP)2(H2O)2 was exposed to NO to establish the accessibility of unsaturated metal centers and crystallographic results show that NO binds to Ni with bent coordination geometry. The adsorption characteristics of CO on isostructural NaNi3(OH)(SIP)2 and NaCo3(OH)(SIP)2 were studied over the temperature range 268–348 K and pressures up to 20 bar. CO surface excess isotherms for NaNi3(OH)(SIP)2 at 348 K were reversible and non-hysteretic for pressures below the isotherm point of inflection. However, above this point, isotherms had both reversible and irreversible adsorption components. The irreversible component remaining adsorbed in ultra-high vacuum at 348 K was 4.9 wt%. Subsequent sequential CO adsorption/desorption isotherms were non-hysteretic and fully reversible. The thermal stability and stoichiometry of the product were investigated by in situ temperature programmed desorption combined with thermogravimetric analysis and mass spectrometry. This gave a discrete CO peak at ∼500 K indicating thermally stable bonding of CO to the framework (0.42 × CO per formula desorbed (2.31 wt%)) and a weaker CO2 peak was observed at 615 K. The remaining adsorbed species were desorbed as a mixture of CO and CO2 overlapping with NaNi3(OH)(SIP)2 framework decomposition. CO physisorption induces structural change, which leads to CO chemisorption on NaNi3(OH)(SIP)2 above the point of inflection in the isotherm, with the formation of a new thermally stable porous framework. The porous structure of the framework was confirmed by CO2 adsorption at 273 K. Therefore, CO chemisorption is attributed to breaking of the hemilabile switchable sulfonate group, while the framework structural integrity is retained by the stable carboxylate linkers. In contrast, studies of CO adsorption on NaCo3(OH)(SIP)2 showed hysteretic isotherms, but no evidence for irreversible chemisorption CO was observed. The CO/N2 selectivity for NaNi3(OH)(SIP)2 and NaCo3(OH)(SIP)2 were 2.4–2.85 (1–10 bar) and 1.74–1.81 (1–10 bar). This is the first demonstration of physisorption driving structural change in a hemilabile porous framework material and demonstrates a transition from physisorption to irreversible thermally stable CO chemisorption.


 Please wait while we load your content...
Please wait while we load your content...