Chemically fluorinated graphene oxide for room temperature ammonia detection at ppb levels†
Abstract
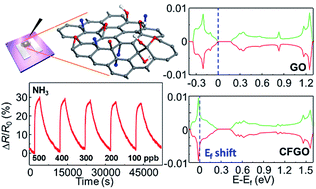
Chemoresistive gas sensors based on two-dimensional (2D) materials including graphene-based materials have attracted significant research interest owing to their potential use in next-generation technologies including the Internet of Things (IoT). The functionalization of 2D materials is considered as a key strategy to achieve superior gas sensing properties such as high selectivity, high sensitivity, and reversible response and recovery, because it can modulate the chemical and electrical properties of 2D materials for more efficient gas sensing. Herein, we present a facile solution process and the room temperature gas sensing properties of chemically fluorinated graphene oxide (CFGO). The CFGO sensors exhibit improved sensitivity, selectivity, and reversibility upon exposure to NH3 with a significantly low theoretical detection limit of ∼6 ppb at room temperature in comparison to NO2 sensing properties. The effect of fluorine doping on the sensing mechanism is examined by first-principles calculations based on density functional theory. The calculations reveal that the fluorine dopant changes the charge distribution on the oxygen containing functional groups in graphene oxide, resulting in the preferred selective adsorption and desorption of NH3 molecules. We believe that the remarkable NH3 sensing properties of CFGO and investigation by first-principles calculations would enlarge the possibility of functionalized 2D materials for practical gas sensing applications such as the IoT.


 Please wait while we load your content...
Please wait while we load your content...