Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution†
Abstract
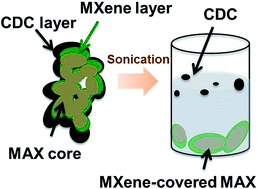
In this study, we successfully demonstrate the electrochemical etching of Al from porous Ti2AlC electrodes in dilute hydrochloric acid to form a layer of Ti2CTx MXene on Ti2AlC. This is the first report on etching of the A layer from the MAX phase in a fluoride-free solution as a less hazardous method to process and handle MXenes. In addition, these MXenes possess only –Cl terminal groups, as well as the common ones, such as –O and –OH. However, electrochemical etching can also result in subsequent over-etching of parent MAX phases to carbide-derived carbon (CDC). We propose a core–shell model to explain electrochemical etching of Ti2AlC to Ti2CTx and CDC. The proposed model suggests that a careful balance in etching parameters is needed to produce MXenes while avoiding over-etching. Our electrochemical approach expands the possible range of both etching techniques and resulting MXene compositions.


 Please wait while we load your content...
Please wait while we load your content...