A self-supported NiMoS4 nanoarray as an efficient 3D cathode for the alkaline hydrogen evolution reaction†
Abstract
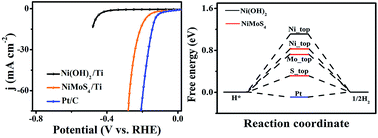
Developing non-noble-metal hydrogen evolution reaction electrocatalysts with high activity is critical for future renewable energy systems. Here we describe the development of a self-supported NiMoS4 nanosheet array on Ti mesh (NiMoS4/Ti) through a facile two-step hydrothermal strategy. As a 3D nanoarray electrode for electrochemical hydrogen evolution, NiMoS4/Ti shows exceptionally high catalytic activity and strong durability in 0.1 M KOH (pH: 13). It needs overpotentials of only 194 and 263 mV to drive geometrical catalytic current densities of 10 and 50 mA cm−2, respectively. Moreover, such a catalyst also demonstrates superior long-term stability with a high turnover frequency of 0.75 mol H2 s−1 at an overpotential of 148 mV. Density functional theory calculations suggest a more favorable hydrogen adsorption free energy on the NiMoS4 surface.


 Please wait while we load your content...
Please wait while we load your content...