Perovskites decorated with oxygen vacancies and Fe–Ni alloy nanoparticles as high-efficiency electrocatalysts for the oxygen evolution reaction†
Abstract
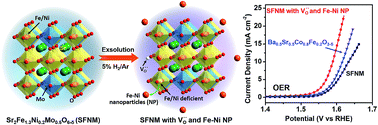
Perovskite oxides have emerged as promising electrocatalysts for the sluggish oxygen evolution reaction (OER) which limits the efficiencies of rechargeable energy storage technologies and hydrogen production from water splitting. Understanding materials characteristics that affect OER activity is of paramount importance for the optimization of perovskite oxides for the OER. Herein, a series of Sr2Fe1.3Ni0.2Mo0.5O6−δ (SFNMs) decorated with oxygen vacancies and Fe–Ni alloy nanoparticles were designed to increase both the number and the reactivity of active sites in the perovskite catalysts. Theoretical calculations reveal that oxygen vacancies have a beneficial effect on the OER by increasing the adsorption energy of H2O, in line with the experimental results that the SFNM sample enriched with oxygen vacancies possesses a high intrinsic OER activity. SFNM decorated with metallic nanoparticles, which was prepared by reducing SFNM in 5% H2/Ar, shows a Tafel slope of 59 mV dec−1 and an OER overpotential of 0.36 V at a current density of 10 mA cm−2. This performance is superior to that of Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) and close to that of commercial IrO2. The outstanding performance is attributed to the fact that the oxygen vacancies together with the exsolved alloy nanoparticles on the perovskite backbone can increase both the number and the reactivity of active sites.


 Please wait while we load your content...
Please wait while we load your content...