Selective electrochemical CO2 reduction over highly porous gold films†
Abstract
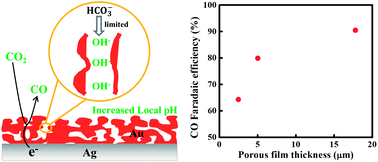
Electrocatalytic reduction of CO2 to CO is usually subject to the competitive reduction of H+ to hydrogen. In this work, it was demonstrated that increasing the local pH at the electrode/electrolyte interface would greatly improve the selectivity for CO2 reduction to CO by inhibiting hydrogen evolution, resulting in a high CO faradaic efficiency of 90.5%. And this pH-induced effect can be achieved by increasing the thickness of the porous gold film through a facile synthetic technique, based on the enhancement of the mass transfer resistance within the highly porous electrode. Moreover, the thickest film, which had a large electrochemical surface area, displayed a significantly improved catalytic activity for CO2 reduction at a low overpotential of 390 mV. These results indicate that increasing the local pH by thickening the porous gold film is selective and efficient for electrochemical CO2 reduction.


 Please wait while we load your content...
Please wait while we load your content...