CO2 capture, storage, and conversion using a praseodymium-modified Ga2O3 photocatalyst†
Abstract
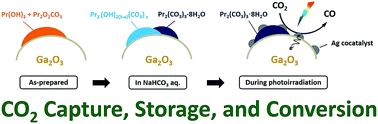
Praseodymium-modified gallium oxide (Pr/Ga2O3) was found to show enhanced activity and selectivity toward CO evolution in the photocatalytic conversion of CO2 using H2O as an electron donor in an aqueous solution of NaHCO3 as compared to those of bare Ga2O3. The as-prepared Pr species, including Pr(OH)3 and Pr2O2CO3, on the surface of Ga2O3 were transformed into Pr hydroxycarbonates (Pr2(OH)2(3−x)(CO3)x) and Pr carbonate hydrates (Pr2(CO3)3·8H2O) in an aqueous solution of NaHCO3, and then the Pr2(OH)2(3−x)(CO3)x was further transformed into Pr2(CO3)3·8H2O by CO2 bubbling under photoirradiation. This indicates that CO2 molecules dissolved in water can be captured and stored using Pr species in an aqueous solution of NaHCO3 under CO2 bubbling. More importantly, Pr2(OH)2(3−x)(CO3)x and Pr2(CO3)3·8H2O accumulated on the surface were decomposed to CO over the Ga2O3 photocatalyst with a Ag cocatalyst. Consequently, Ag/Pr/Ga2O3 exhibits much higher activity (249 μmol h−1 of CO) than the pristine Ag-loaded Ga2O3 (136 μmol h−1 of CO).


 Please wait while we load your content...
Please wait while we load your content...