Composition design, optical gap and stability investigations of lead-free halide double perovskite Cs2AgInCl6†
Abstract
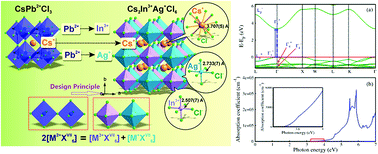
The discovery of lead-free double perovskites provides a feasible way of searching for air-stable and environmentally benign solar cell absorbers. Herein we report the design and hydrothermal crystal growth of double perovskite Cs2AgInCl6. The crystal structure, morphology related to the crystal growth habit, band structure, optical properties, and stability are investigated in detail. This perovskite crystallized in a cubic unit cell with the space group Fm3![[> with combining macron]](https://www.rsc.org/images/entities/char_003e_0304.gif) m and is composed of [AgCl6] and [InCl6] octahedra alternating in a ordered rock-salt structure, and the as-obtained crystal size is dependent on the hydrothermal reaction time. Cs2AgInCl6 is a direct gap semiconductor with a wide band gap of 3.23 eV obtained experimentally and 3.33 eV obtained by DFT calculation. This theoretically predicted and experimentally confirmed optical gap is a prototype of the band gaps that are direct and optically allowed except at the single high-symmetry k-point, which didn't raise interest before but have potential applications in future technologies. Cs2AgInCl6 material with excellent moisture, light and heat stability shows great potential for photovoltaic and other optoelectronic applications via further band gap engineering.
m and is composed of [AgCl6] and [InCl6] octahedra alternating in a ordered rock-salt structure, and the as-obtained crystal size is dependent on the hydrothermal reaction time. Cs2AgInCl6 is a direct gap semiconductor with a wide band gap of 3.23 eV obtained experimentally and 3.33 eV obtained by DFT calculation. This theoretically predicted and experimentally confirmed optical gap is a prototype of the band gaps that are direct and optically allowed except at the single high-symmetry k-point, which didn't raise interest before but have potential applications in future technologies. Cs2AgInCl6 material with excellent moisture, light and heat stability shows great potential for photovoltaic and other optoelectronic applications via further band gap engineering.


 Please wait while we load your content...
Please wait while we load your content...