The cycling stability of the in situ formed Mg-based nanocomposite catalyzed by YH2†
Abstract
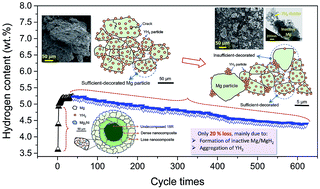
Long cycling life is one prerequisite for the commercial application of hydrogen storage materials. The cycling stability of a promising Mg + Mg2Ni + YH2 hydrogen storage nanocomposite made by hydrogen-induced decomposition of the 18R-type long period stacking ordered (LPSO) structure is investigated. At 300 °C, it absorbs maximum ∼5.2 wt% H at the 40th de/hydrogenation cycle and still has 4.3 wt% H even after 620 cycles. Both activation and passivation occur during the 40th–620th cycles, where the absorption rate within 0–15 s becomes faster but the rate after 15 s gradually slows down. Characterizations by synchrotron X-ray powder diffraction and transmission electron microscopy reveal that this phenomenon is closely related to the pulverization of particles and the aggregation of YH2 nanocatalysts. From the first-principles calculations, the catalytic effect of YH2 is ascribed to the relatively high interfacial energy of YH2/Mg, the low diffusion energy barrier for H at the YH2/Mg interface, and the high affinity between YH2 and H. 17% loss of hydrogen capacity is attributed to the formation of kinetically inactive Mg/MgH2 phases, the aggregation of YH2 and the oxidation of Mg. Minimizing the separation between the Mg/MgH2 matrix and YH2 nanocatalysts is crucial to maintain the high effective capacity of this nanocomposite.


 Please wait while we load your content...
Please wait while we load your content...