Mn3O4 nanoparticles on layer-structured Ti3C2 MXene towards the oxygen reduction reaction and zinc–air batteries†
Abstract
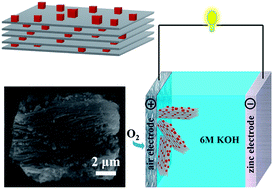
Non-precious metal catalysts, such as manganese oxide (Mn3O4), with efficient activity and superior stability are highly required. However, poor conductivity, dissolution, and high cohesion significantly limit the performance of Mn3O4 nanoparticles as an electrode material. Herein, Mn3O4 nanoparticles supported on layered Ti3C2 MXene (Mn3O4/MXene) nanocomposite with superb oxygen reduction reaction performance have been reported for the first time. The as-prepared Mn3O4/MXene nanocomposite display favorable electrochemical activity in oxygen reduction reaction with a dominant four-electron oxygen reduction pathway and an onset potential at 0.89 V (same as that of Pt/C) in an alkaline solution. Layered MXenes exhibit metal conductivity as well as hydrophilicity, which would greatly improve the chemical properties of the nano-sized particles by inhibiting aggregation and increasing the electron transfer speed. Furthermore, Mn3O4/MXene exhibits higher stability than Mn3O4/acetylene black owing to the highly stable Mn3O4/MXene hybrid structure. The as-prepared Zn–air battery based on the Mn3O4/MXene air-cathode exhibits a high open potential (1.37 V), superb power density (150 mW cm−2), and excellent stability (no obvious potential change in 100 hours). The prepared low-cost Mn3O4/MXene nanocomposite with superior oxygen reduction reaction performance can be a promising candidate as an oxygen reduction reaction catalyst and for use in zinc–air batteries and other energy storage devices.


 Please wait while we load your content...
Please wait while we load your content...