Ternary nickel–iron sulfide microflowers as a robust electrocatalyst for bifunctional water splitting†
Abstract
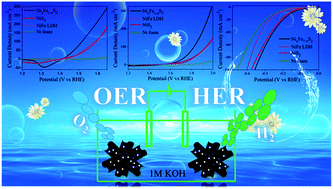
The development of low-cost, highly efficient, and stable electrocatalysts toward overall water splitting (both the anodic oxygen evolution reaction (OER) and the cathodic hydrogen evolution reaction (HER)) is essential for future energy supply. In this report, 3D ternary nickel iron sulfide microflowers with a hierarchically porous structure directly grown on Ni foam via a convenient two-step method have been fabricated, and further used as an efficient electrocatalyst for both the HER and the OER. Thanks to the unique 3D morphology and strong electron interactions between Fe, Ni and S, the as-synthesized Ni0.7Fe0.3S2 delivers an overpotential of 198 mV at a current density of 10 mA cm−2 toward the OER in alkaline media, which is, to the best of our knowledge, the best among the reported non-noble metal based electrocatalysts. Furthermore, when used as both the anode and cathode, a low cell voltage of 1.625 V is needed to obtain 10 mA cm−2 for the overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...