Subtle side-chain tuning on terminal groups of small molecule electron acceptors for efficient fullerene-free polymer solar cells†
Abstract
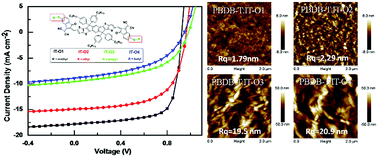
Four dithienoindaceno[1,2-b:5,6-b′]dithiophene (DT-IDT) based small molecules IT-O1, IT-O2, IT-O3 and IT-O4 with increasing alkoxyl chain length from methoxy to butoxy on the terminal-groups were synthesized to investigate the end-group side-chain effects on these acceptor–donor–acceptor-type small molecule electron acceptors. The optical absorption and energy levels of the four molecules, blend morphologies, carrier mobilities, and photovoltaic performances of the devices blended with the polymer PBDB-T are systematically investigated. Interestingly, both the solubility and electron mobility are enhanced for these materials with decrease in side chain length, which lead to ideal morphologies and balanced charge transport. Hence, increased Jsc and FF, and thus distinctly higher PCEs of 11.6% were obtained from the PBDB-T:IT-O1-based devices.


 Please wait while we load your content...
Please wait while we load your content...