Enhanced specific heat capacity of binary chloride salt by dissolving magnesium for high-temperature thermal energy storage and transfer
Abstract
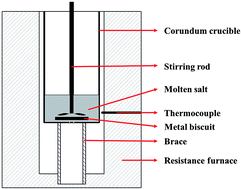
Thermal energy storage and transfer technology has received significant attention with respect to concentrating solar power (CSP) and industrial waste heat recovery systems. In this study, we report a novel method to synthesize nanofluids by dissolving magnesium metal in NaCl–CaCl2 eutectic molten salt to enhance the specific heat capacity without the conventional agglomerate effect of nanoparticles. It was found that the solubility of magnesium in the binary molten salt reached 0.075% and 0.185% at 550 °C and 750 °C, respectively. Magnesium did not react with the molten salt but dissolved in it in the form of liquid magnesium metal and did not change the melting temperature of the binary molten salt. The liquid specific heat capacities of nanofluids containing 1.0 wt% and 2.0 wt% magnesium were 1.12 J g−1 °C−1 and 1.15 J g−1 °C−1, which were 105.66% and 108.49% higher than those of the binary chloride salts. Magnesium decreased the upper temperature limit and thermal stability, and the nanofluid was thermally and chemically stable after 50 heating/cooling cycles. These results implied that the resulting nanofluid is a promising candidate material for high-temperature heat storage and transfer applications.


 Please wait while we load your content...
Please wait while we load your content...