Electrochemical reactions in fluoride-ion batteries: mechanistic insights from pair distribution function analysis†
Abstract
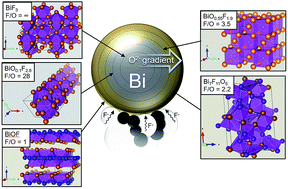
Detailed analysis of electrochemical reactions occurring in rechargeable Fluoride-Ion Batteries (FIBs) is provided by means of synchrotron X-ray diffraction (XRD) and Pair Distribution Function (PDF) analysis. In this study, a symmetrical cell containing Bi–BiF3 composite electrodes was characterized after three cycles. As for Li conversion-based electrodes, our study confirms that a multi-electron redox process occurs between Bi metal and BiF3. XRD and PDF analyses show that multiple Bi oxyfluoride phases are formed due to the presence of Bi oxide in the initial Bi electrode and/or Bi oxyfluoride in the initial BiF3 electrode. Quantitative PDF analysis suggests that oxygen does not migrate through the electrolyte and that the fluoride ion is the sole charge transfer anion. Hence, we showed that oxide species do not prevent the electrochemical reactions, opening the path for the study of metal oxyfluorides as active materials for FIBs.


 Please wait while we load your content...
Please wait while we load your content...