Effect of synthesis temperature on the structural defects of integrated spinel-layered Li1.2Mn0.75Ni0.25O2+δ: a strategy to develop high-capacity cathode materials for Li-ion batteries†
Abstract
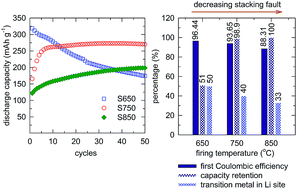
An integrated layered-spinel with a nominal composition of (1 − x)Li1.2Mn0.6Ni0.2O2·xLiMn1.5Ni0.5O4 (0.15 < x < 0.3) was synthesized by a hydrothermal reaction followed by firing at different temperatures. The effects of firing temperature on the phase components, cation disorder, and crystal defects, and their relationship with the electrochemical performance of the cathode material were studied. The sample fired at 650 °C showed the highest capacity of up to 320 mA h g−1 and highest initial coulombic efficiency (98%, 2–4.9 V), but the capacity decreased dramatically to only 55% after 50 cycles. The sample fired at 850 °C showed the slowest activation of the layered phase, requiring up to several dozen cycles. The intermediate firing temperature of 750 °C showed a balance between the activation rate, capacity, initial coulombic efficiency, and cycling stability, with 270 mA h g−1 after 10 cycles and a 99% capacity retention after 50 cycles. All samples showed different rates of the layered-to-spinel phase transformation, which depends on the activation rate. This study reports the relationships between synthesis conditions, structure, and electrochemical performance, providing a strategy to develop high-capacity cathode materials based on the (1 − x)Li1.2Mn0.6Ni0.2O2·xLiMn1.5Ni0.5O4 system.


 Please wait while we load your content...
Please wait while we load your content...