A solothiocarbonyl quinacridone with long chains used as a fluorescent tool for rapid detection of Hg2+ in hydrophobic naphtha samples†
Abstract
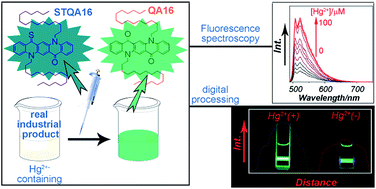
The rapid detection of heavy metal ions in industrial products has gradually garnered great attention, due to concerns about sustainability and the environment. Because of their hydrophobic properties, it is still a big challenge to monitor hazardous impurities found in many industrial products, such as petroleum chemicals and fine chemicals. In this work, a quinacridone-based fluorescent sensor (STQA16) was designed and synthesized for detecting the most toxic metal ion (Hg2+) in real naphtha samples. One carbonyl group on the quinacridone skeleton was selectively thiolated, giving it the ability to interact with Hg2+ and release an emission associated with the quenching of the parent quinacridone efficiently. Two n-hexadecyl chains were introduced into the quinacridone chromophore, which showed improved solubility even in nonpolar hexane solution. The results of both the absorption and emission titration experiments suggested a rapid sensing process, within the first 60 seconds. Furthermore, a real sample detection experiment was performed in naphtha samples and a high detection limit was obtained (1.4 × 10−7 M), because the emission of quinacridone was longer than the background fluorescence of real naphtha samples. To further verify the viability of the method, a recovery experiment was carried out to give a rapid and satisfactory result.


 Please wait while we load your content...
Please wait while we load your content...